Research topics
Cardiovascular disease, type 2 diabetes and obesity are often associated in the same individual, in a particular context known as (cardio)-metabolic syndrome. The latter affects over a quarter of the world's population, and 90% of type 2 diabetics. The metabolic syndrome brings together physiological and metabolic abnormalities (insulin resistance, hypertension, dyslipidemia, obesity), which are conducive to cardiovascular events (3-fold increase), type 2 diabetes (5-fold increase) and all-cause mortality (1.5-fold increase) (Mottillo et al., J Am Coll Cardiol, 2010). It goes hand in hand with the sharp rise in diabetes prevalence, estimated to affect 537 million people worldwide (IDF Atlas, 2022), and the increase in cardiovascular disease-related mortality, estimated to account for 30% of causes of death worldwide (GBD 2015 Mortality and Causes of Death Collaborators, Lancet, 2016). On the other hand, the pathophysiological mechanisms underlying the development of metabolic syndrome and associated risk factors are still poorly understood.
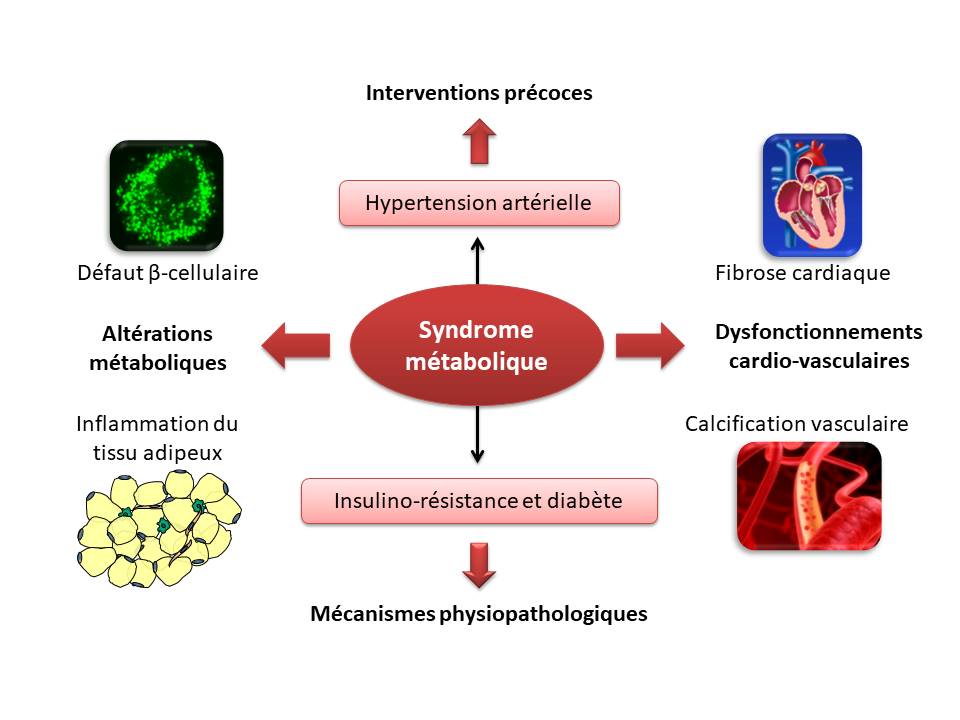
The Unit's project focuses on biocommunication between the cardiovascular/renal axis and metabolic tissues, building on the results generated and know-how acquired during the previous five-year period. Metabolic dysfunction in animals, either of genetic origin (Zucker and ZDF), or of nutritional origin (fructose diet or HFD), or in humans, will be the common denominator in the lines of research developed below. In this particular context, we will investigate the mechanisms involved in certain cardiovascular dysfunctions (fibrosis, calcification), as well as in metabolic alterations (inflammation of adipose tissue and β-cellular function).
Three axes are therefore developed:
-
Cardiac fibrosis and vascular calcification in metabolic dysfunction:
Our aim is to gain a better understanding of the molecular mechanisms involved in the development of cardiac fibrosis (extracellular matrix, microbiota) and vascular calcification (cytokines, RAGE pathway) that occur during metabolic dysfunction, whether or not associated with hypertension and renal failure.
-
Biocommunication between adipose tissue and pancreatic β-cell in metabolic dysfunction:
Our aim is to investigate how factors produced by visceral and peripancreatic adipose tissue (lipid derivatives, cytokines, chemokines, miRNA) may influence pancreatic β-cell function during the development of type 2 diabetes.
-
Screening and evaluation of new compounds of therapeutic interest:
Our aim here is to identify either substances of natural origin, or compounds derived from synthetic chemistry, which could have beneficial activity against the metabolic and/or cardiovascular disorders present in type 2 diabetes.