Free Radical Biology and Medicine
Jonas Laget, Claire Vigor, Agathe Nouvel, Amandine Rocher, Jérémy Leroy, Laura Jeanson, Guillaume Reversat, Camille Oger, Jean-Marie Galano, Thierry Durand, Sylvie Péraldi-Roux, Jacqueline Azay-Milhau, Anne-Dominique Lajoix. Reduced production of isoprostanes by peri-pancreatic adipose tissue from Zucker fa/fa rats as a new mechanism for β-cell compensation in insulin resistance and obesity. Free Radical Biology and Medicine, 2022, 182: 160-170.
https://www.sciencedirect.com/science/article/pii/S0891584922000661
Abstract
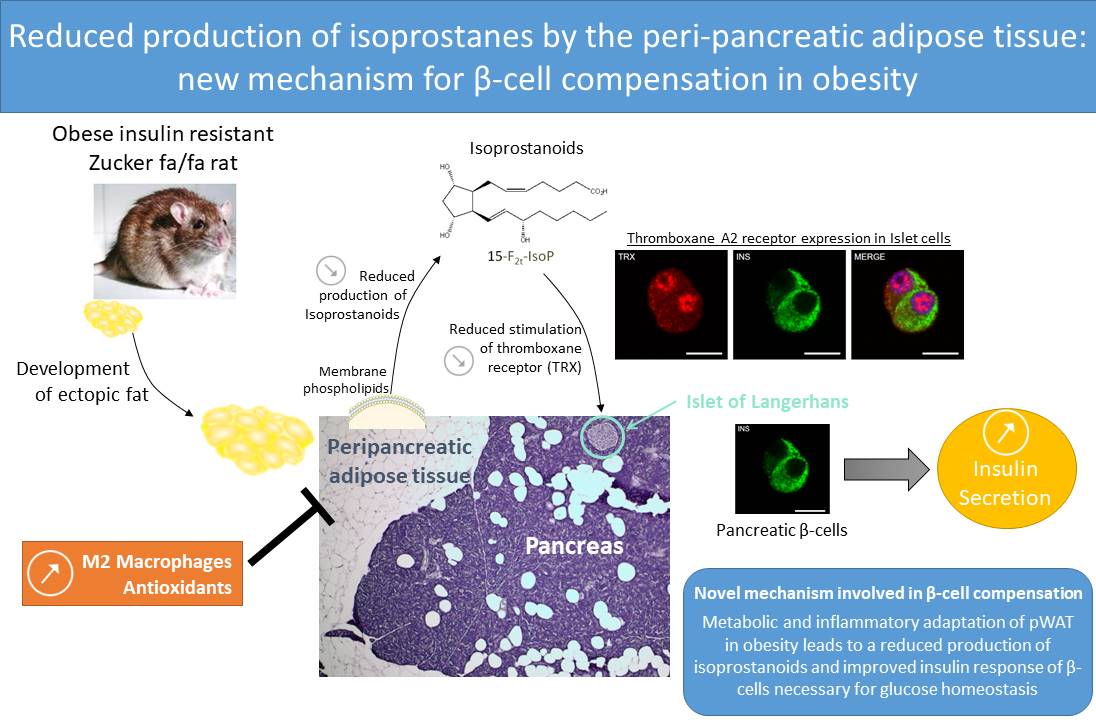
During early stages of type 2 diabetes, named prediabetes, pancreatic β-cells compensate for insulin resistance through increased insulin secretion in order to maintain normoglycemia. Obesity leads to the development of ectopic fat deposits, among which peri-pancreatic white adipose tissue (pWAT) can communicate with β-cells through soluble mediators. Thus we investigated whether pWAT produced oxygenated lipids, namely isoprostanes and neuroprostanes and whether they can influence β-cell function in obesity. In the Zucker fa/fa rat model, pWAT and epididymal white adipose tissue (eWAT) displayed different inflammatory profiles. In obese rats, pWAT, but not eWAT, released less amounts of 5-F2t-isoprostanes, 15-F2t-isoprostanes, 4-F4t-neuroprostanes and 10-F4t-neuroprostane compared to lean animals. These differences could be explained by a greater induction of antioxidant defenses enzymes such as SOD-1, SOD-2, and catalase in pWAT of obese animals compared to eWAT. In addition, sPLA2 IIA, involved in the release of isoprostanoids from cellular membranes, was decreased in pWAT of obese animals, but not in eWAT, and may also account for the reduced release of oxidized lipids by this tissue. At a functional level, 15-F2t-isoprostane epimers, but not 5-F2t-isoprostanes, were able to decrease glucose-induced insulin secretion in pancreatic islets from Wistar rats. This effect appeared to be mediated through activation of the thromboxane A2 receptor and reduction of cAMP signaling in pancreatic islets. In conclusion, through the removal of an inhibitory tone exerted by isoprostanes, we have shown, for the first time, a new mechanism allowing β-cells to compensate for insulin resistance in obesity that is linked to a biocommunication between adipose tissue and β-cells.
Graphical abstract